Ketamine shows promise in treating Alzheimer’s Disease, Parkinson’s Disease as well as depression, suicide ideation and more
In 2021, Silo Pharma (OTCQB: SILO) entered into a joint venture with Zylö Therapeutics to “explore the clinical development of ketamine using ZTI’s Z-pod (transdermal) technology.” This innovative technology allows Silo, an early-stage biopharmaceutical company, to develop a ketamine delivery system for those suffering dysphagia, a difficulty or complete inability to swallow.(1) Silo Pharma is exploring the efficacy of ketamine in treating patients suffering from both Alzheimer’s Disease (“AD”) and Parkinson’s Disease (“PD”). Over 80% of PD patients(2) and 75% of AD patients(3) experience dysphagia.
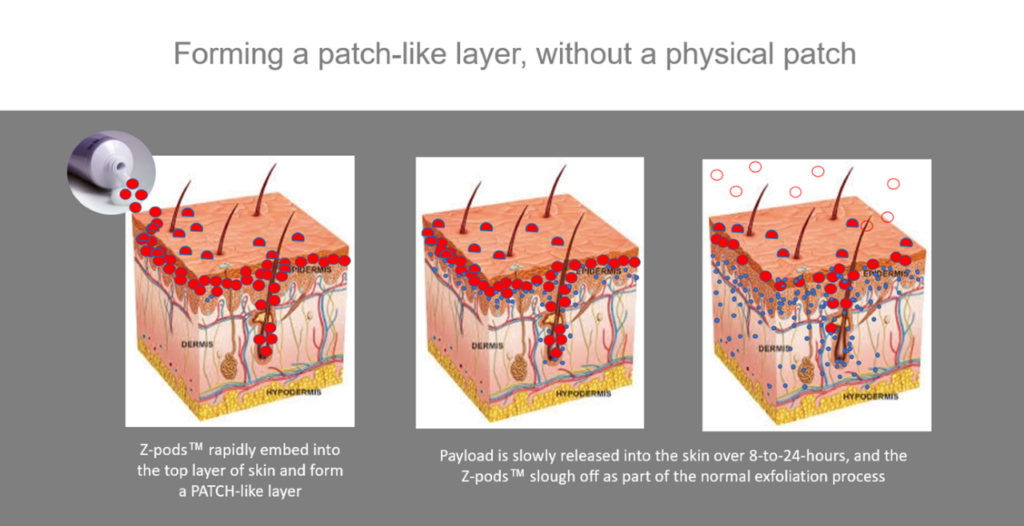
According to Dr. Jay Blankenship, Zylö Therapeutics V.P. of Business Development, “ketamine is an incredibly versatile drug that continues to show promise with new therapeutic indications.” Eric Weisblum, CEO of Silo Pharma believes “the ability to deliver ketamine (through the skin) in a time-released manner will allow us to explore both the safety and efficacy of our therapeutics … We believe that Zylo’s patented technology will allow us to reach potential patients that suffer with dysphagia.” Currently, ketamine is administered intravenously, via bone tissue (intraosseous), intramuscularly, orally or intranasally (through the nose), methods that are impractical, painful and sometimes subject to nausea.(4)
Ketamine has been available for over “half a century … first synthesized in 1962 by Calvin Stevens at Parke-Davis Co (now Pfizer) as an alternative anesthetic to phencyclidine.” Ketamine facilitates “hypnotic (sleep-producing), analgesic (pain-relieving) and amnesic (short-term memory loss) effects; no other drug used in clinical practice produces these three important effects at the same time. Ketamine is a standard in operating rooms. Due to its “bronchial dilatory profile,” ketamine is the safer choice for patients with “asthma and life-threatening acute bronchial constriction,” and is “preferred in patients with unstable hemodynamics (blood flow). Ketamine is also one of the very few drugs approved for anesthesia induction in caesarean sections. It is considered the agent of choice in children and burn victims.”(5)
Novel ketamine uses show promise in treating PD-related depression as well as dyskinesia, resulting from the side effects of levodopa treatments. Dyskinesia includes involuntary, erratic, writhing movements of the face, arms, legs, and/or trunk.
“Recent clinical findings suggest that ketamine may provide neuroprotection and reduce neuropsychiatric symptoms associated with AD.”(6) Neuropsychiatric symptoms include agitation, depression, apathy, delusions, hallucinations, and sleep impairment. Ketamine has also been studied to battle suicide ideation, as well as “eating disorders, problematic substance use, post-traumatic stress and anxiety…”(7)
In October 2021, Silo entered into a sponsored research agreement with Columbia University incorporating the work of Dr. Christine Ann Denny. According to a Silo Pharma news release, “Dr. Denny’s pioneering research into whether ketamine … may improve memory retrieval, halt, or even reverse, the process of Alzheimer’s disease-related to memory loss or cognitive aging, could have life-altering implications for people suffering with everything from Alzheimer’s disease to post-traumatic stress disorder (‘PTSD’).”
Weisblum said, “to be able to bring hope and possibly a therapeutic to patients suffering from Alzheimer’s disease (and potentially other ailments) is an exciting proposition for Silo Pharma. The unique compounds being developed at Columbia have shown great promise, and we look forward to continuing to explore and develop these therapeutics.”
Illustration courtesy of Zylö Therapeutics.
For Silo Pharma’s most recent news, click here.
Silo Pharma
560 Sylvan Avenue, Suite 3160
Englewood Cliffs, NJ 07632
(1) https://www.nidcd.nih.gov/health/dysphagia
(2) Suttrup, I., Warnecke, T. Dysphagia in Parkinson’s Disease. Dysphagia 31, 24–32 (2016). https://doi.org/10.1007/s00455-015-9671-9
(3) Yaprak Seçil, Şehnaz Arıcı, Tülay Kurt İncesu, Nevin Gürgör, Yeşim Beckmann, CumhurErtekin, 2016, Neurophysiologie Clinique/Clinical Neurophysiology
(4) Ketamine use in current clinical practice
Mei Gao, Damoon Rejaei, Hong Liu
Acta Pharmacol Sin. 2016 Jul; 37(7): 865–872. Published online 2016 Mar 28. doi: 10.1038/aps.2016.5
(5) Ibid
(6) Mohammad Shehata I, Masood W, Nemr N, Anderson A, Bhusal K, Edinoff AN, Cornett EM, Kaye AM, Kaye AD. The Possible Application of Ketamine in the Treatment of Depression in Alzheimer’s Disease. Neurology International. 2022; 14(2):310-321. https://doi.org/10.3390/neurolint14020025
(7) University of British Columbia Okanagan campus. “Exploring the therapeutic uses of ketamine: The drug could be a game changer in the treatment of mental illness.” ScienceDaily. ScienceDaily, 18 March 2022. <www.sciencedaily.com/releases/2022/03/220318161446.htm>.
For more information, visit the company’s website at www.SiloPharma.com.
NOTE TO INVESTORS: The latest news and updates relating to SILO are available in the company’s newsroom at https://ibn.fm/SILO
About InvestorWire
InvestorWire is the wire service that gives you more. From regional releases to global announcements presented in multiple languages, we offer the wire-grade dissemination products you’ll need to ensure that your next press release grabs the attention of your target audience and doesn’t let go. While our competitors look to nickel and dime you with hidden fees and restrictive word limits, InvestorWire keeps things transparent. We offer UNLIMITED Words on all domestic releases. While other wire services may provide a basic review of your release, InvestorWire helps you put your best foot forward with complimentary Press Release Enhancement.
With our competitors, the work is done the second your release crosses the wire. Not with InvestorWire. We include follow-up coverage of every release by leveraging the ever-expanding audiences of the 50+ brands that make up the InvestorBrandNetwork.
Get more out of your next press release with InvestorWire. It’s unlike anything you’ve seen before.
For more information, please visit https://www.investorwire.com
Please see full terms of use and disclaimers on the InvestorBrandNetwork website applicable to all content provided by IBN, wherever published or re-published: http://ibn.fm/Disclaimer
InvestorWire (IW)
8033 Sunset Blvd Suite 1037-IW
Los Angeles, CA 90046
310.299.1717 Office
www.investorwire.com
[email protected]
InvestorWire is part of the InvestorBrandNetwork.
